Single-crystal X-ray Diffraction Instrumentation - How Does It Work?
X-ray diffractometers consist of three basic elements, an X-ray tube, a sample holder, and an X-ray detector. X-rays are generated in a cathode ray tube by heating a filament to produce electrons, accelerating the electrons toward a target by applying a voltage, and impact of the electrons with the target material. When electrons have sufficient energy to dislodge inner shell electrons of the target material, characteristic X-ray spectra are produced. These spectra consist of several components, the most common being Kα and Kβ. Kα consists, in part, of Kα1 and Kα2. Kα1 has a slightly shorter wavelength and twice the intensity as Kα2. The specific wavelengths are characteristic of the target material. Filtering, by foils or crystal monochrometers, is required to produce monochromatic X-rays needed for diffraction. Kα1and Kα2 are sufficiently close in wavelength such that a weighted average of the two is used. Molybdenum is the most common target material for single-crystal diffraction, with MoKα radiation = 0.7107Å. These X-rays are collimated and directed onto the sample. When the geometry of the incident X-rays impinging the sample satisfies the Bragg Equation, constructive interference occurs. A detector records and processes this X-ray signal and converts the signal to a count rate which is then output to a device such as a printer or computer monitor. X-rays may also be produced using a synchotron, which emits a much stronger beam.
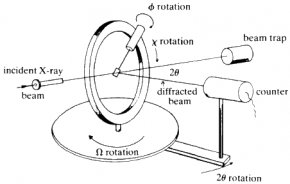
Single-crystal diffractometers use either 3- or 4-circle goniometers. These circles refer to the four angles (2θ, χ, φ, and Ω) that define the relationship between the crystal lattice, the incident ray and detector. Samples are mounted on thin glass fibers which are attached to brass pins and mounted onto goniometer heads. Adjustment of the X, Y and Z orthogonal directions allows centering of the crystal within the X-ray beam.
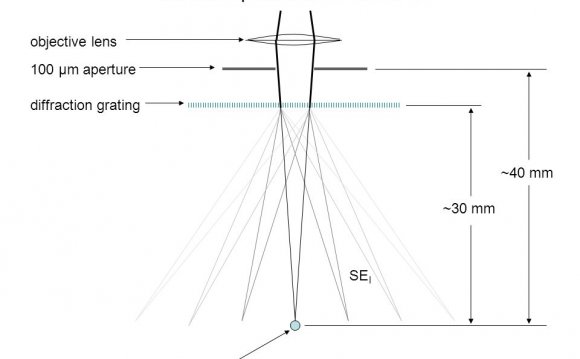
X-rays leave the collimator and are directed at the crystal. Rays are either transmitted through the crystal, reflected off the surface, or diffracted by the crystal lattice. A beam stop is located directly opposite the collimator to block transmitted rays and prevent burn-out of the detector. Reflected rays are not picked up by the detector due to the angles involved. Diffracted rays at the correct orientation for the configuration are then collected by the detector.
 Modern single-crystal diffractometers use CCD (charge-coupled device) technology to transform the X-ray photons into an electrical signal which are then sent to a computer for processing.
Modern single-crystal diffractometers use CCD (charge-coupled device) technology to transform the X-ray photons into an electrical signal which are then sent to a computer for processing.
Applications
Single-crystal X-ray diffraction is most commonly used for precise determination of a unit cell, including cell dimensions and positions of atoms within the lattice. Bond-lengths and angles are directly related to the atomic positions. The crystal structure of a mineral is a characteristic property that is the basis for understanding many of the properties of each mineral. Specific applications of single-crystal diffraction include:
- New mineral identification, crystal solution and refinement
- Determination of unit cell, bond-lengths, bond-angles and site-ordering
- Characterization of cation-anion coordination
- Variations in crystal lattice with chemistry
- With specialized chambers, structures of high pressure and/or temperature phases can be determined
- Determination of crystal-chemical vs. environmental control on mineral chemistry
- Powder patterns can also be derived from single-crystals by use of specialized cameras (Gandolfi)
Strengths
- No separate standards required
- Non-destructive
- Detailed crystal structure, including unit cell dimensions, bond-lengths, bond-angles and site-ordering information
- Determination of crystal-chemical controls on mineral chemistry
Limitations
- Must have a single, robust (stable) sample, generally between 50—250 microns in size
- Optically clear sample
- Twinned samples can be handled with difficulty
- Data collection generally requires between 24 and 72 hours
User's Guide - Sample Collection and Preparation
Sample Selection and Preparation
Samples for single-crystal diffraction should be selected from unfractured, optically clear crystals. This can be determined by viewing the samples under crossed polars on a petrographic microscope. Crystals can be broken off a larger sample and the best fragment selected. Samples should be between 30 and 300 microns, with ideal crystals averaging 150-250 microns in size. To minimize absorption affects, equant crystals are preferred. Spherical crystals can be created using a small, air-powered crystal tumbler, however easily cleaved minerals can break during this process. Therefore, minerals lacking cleavage are the best choice for this step. If the sample is inequant, this must be corrected for during absorption corrections to the data.
Goniometer head.
Provenance: Image courtesy of Sean Parkin, University of Kentucky Dept. of Chemistry.
Sample MountingRELATED VIDEO










